Stereochemistry of the E2 Reaction. E2 elimination reactions of certain isomeric cycloalkyl halides show unusual rates and regioselectivity that are not explained by the principles thus far discussed. For example, trans-2-methyl-1-chlorocyclohexane reacts with alcoholic KOH at a much slower rate than does its cis-isomer.
How to Predict the Stereochemistry Outcome of an E2 Reaction Help Me With Organic Chemistry! – YouTube
Answer and Explanation: 1 Become a Study.com member to unlock this answer! Create your account View this answer 1. The stereochemical outcome of the E2 reaction is given below. The Newman
Source Image: chem.libretexts.org
Download Image
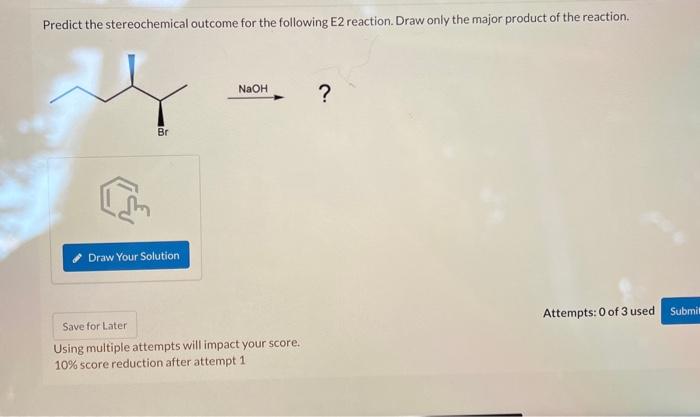
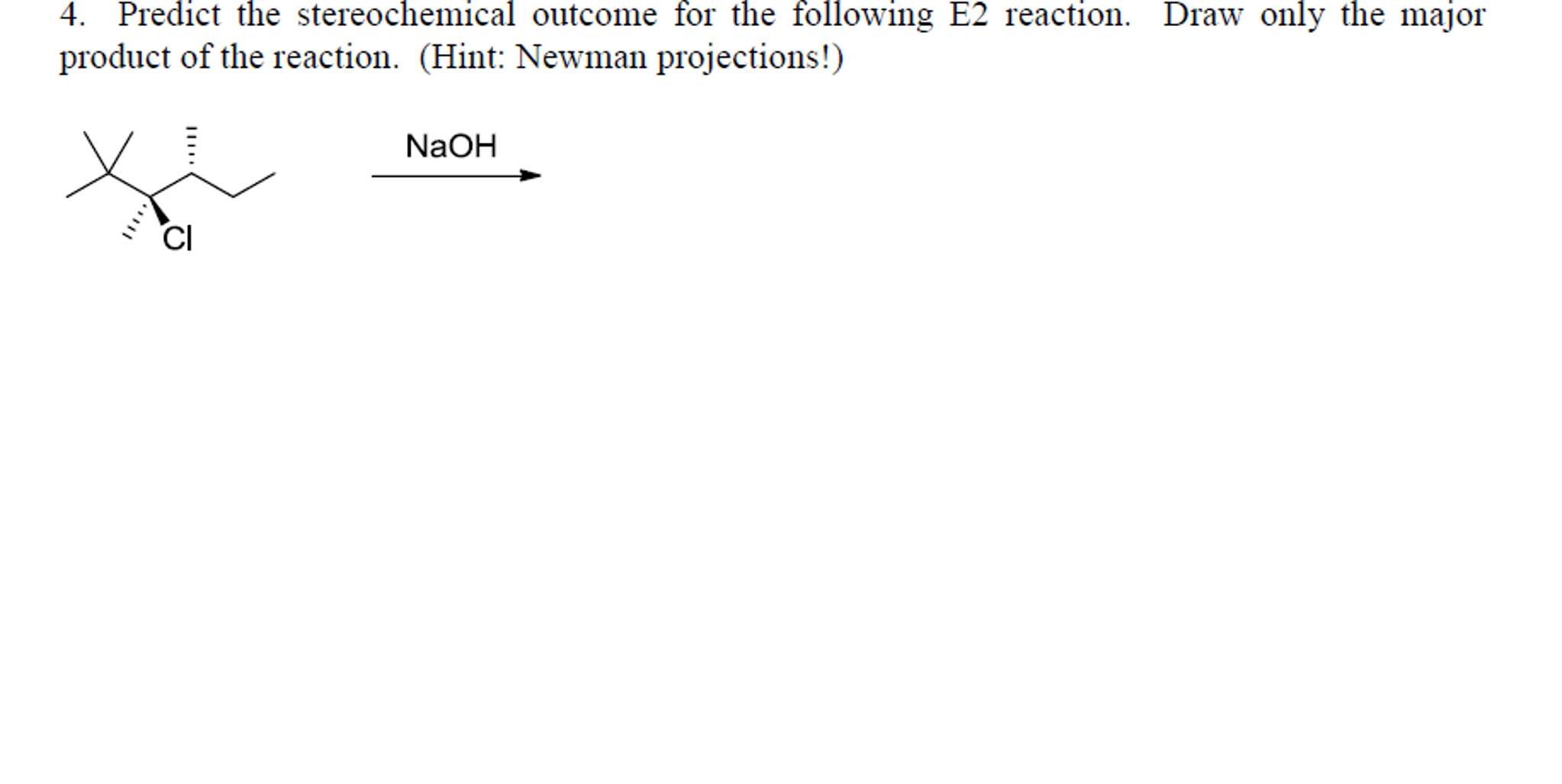
Science Chemistry Chemistry questions and answers Predict the stereochemical outcome for the following E2 reaction. Draw only the major product of the reaction. NaOH ? This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer
Source Image: bartleby.com
Download Image
Solved] ES Practice Problem 07.63 W Ob Identify the sole product of the… | Course Hero Explain the stereochemical outcome of the E2 mechanism Predict the outcome of E2 reactions. Making Alkenes: 2 Possible Pathways All at Once One Step at A Time. E2 Fundamentals Transition State* How Do We Know? Kinetics rate = k [R-X] [ Base] RDS 2nd order Intermediates
Source Image: chegg.com
Download Image
Predict The Stereochemical Outcome For The Following E2 Reaction
Explain the stereochemical outcome of the E2 mechanism Predict the outcome of E2 reactions. Making Alkenes: 2 Possible Pathways All at Once One Step at A Time. E2 Fundamentals Transition State* How Do We Know? Kinetics rate = k [R-X] [ Base] RDS 2nd order Intermediates Predict the stereochemical outcome of the following E2 reaction: Organic Chemistry 9th Edition ISBN: 9781305080485 Author: John E. McMurry Publisher: John E. McMurry Chapter11: Reactions Of Alkyl Halides: Nucleophilic Substitutions And Eliminations Section11.8: The E2 Reaction And The Deuterium Isotope Effect Problem 18P See similar textbooks
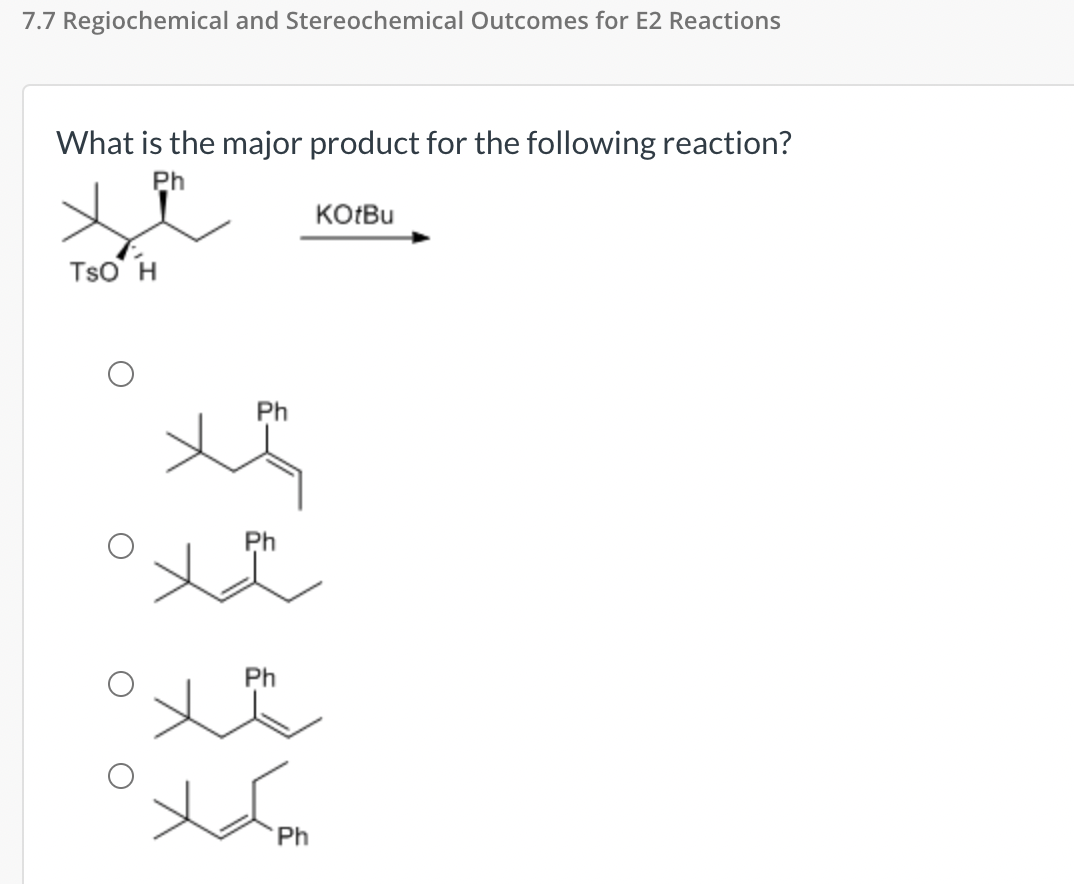
Solved 7.7 Regiochemical and Stereochemical Outcomes for E2 | Chegg.com
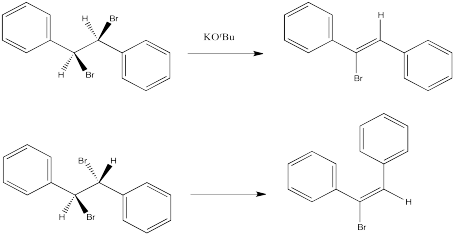
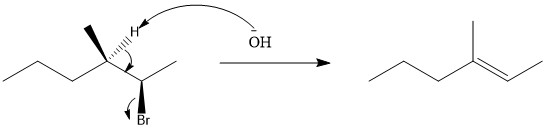
To quickly predict the correct stereoisomer of a stereospecific E2 reaction check the wedge and dash of the beta hydrogen and the leaving group: If one is a wedge and the other one is dash, then it is good to go – simply erase them and place a double bond between these two carbons in the corresponding alkene. Solved Predict the stereochemical outcome for the following | Chegg.com
Source Image: chegg.com
Download Image
Solved Predict the stereochemical outcome for the following | Chegg.com To quickly predict the correct stereoisomer of a stereospecific E2 reaction check the wedge and dash of the beta hydrogen and the leaving group: If one is a wedge and the other one is dash, then it is good to go – simply erase them and place a double bond between these two carbons in the corresponding alkene.
Source Image: chegg.com
Download Image
How to Predict the Stereochemistry Outcome of an E2 Reaction Help Me With Organic Chemistry! – YouTube Stereochemistry of the E2 Reaction. E2 elimination reactions of certain isomeric cycloalkyl halides show unusual rates and regioselectivity that are not explained by the principles thus far discussed. For example, trans-2-methyl-1-chlorocyclohexane reacts with alcoholic KOH at a much slower rate than does its cis-isomer.
Source Image: youtube.com
Download Image
Solved] ES Practice Problem 07.63 W Ob Identify the sole product of the… | Course Hero Science Chemistry Chemistry questions and answers Predict the stereochemical outcome for the following E2 reaction. Draw only the major product of the reaction. NaOH ? This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer
Source Image: coursehero.com
Download Image
Solved 4. Predict the stereo chemical outcome of the | Chegg.com Ernest Zinck. It is the nature of the α carbon that determines the type of substitution. If you have a 3° carbon, the substitution reaction will be SN1. For 1° and 2° carbons, the substitution will be SN2. It is the strength of the base that determines the type of elimination. If you have a strong base, you will get E2 elimination.
Source Image: chegg.com
Download Image
Stereospecificity of E2 Elimination Reactions – Chemistry Steps Explain the stereochemical outcome of the E2 mechanism Predict the outcome of E2 reactions. Making Alkenes: 2 Possible Pathways All at Once One Step at A Time. E2 Fundamentals Transition State* How Do We Know? Kinetics rate = k [R-X] [ Base] RDS 2nd order Intermediates

Source Image: chemistrysteps.com
Download Image
Predicting the Outcome of E2 Reactions Part 1 v2 – YouTube Predict the stereochemical outcome of the following E2 reaction: Organic Chemistry 9th Edition ISBN: 9781305080485 Author: John E. McMurry Publisher: John E. McMurry Chapter11: Reactions Of Alkyl Halides: Nucleophilic Substitutions And Eliminations Section11.8: The E2 Reaction And The Deuterium Isotope Effect Problem 18P See similar textbooks

Source Image: youtube.com
Download Image
Solved Predict the stereochemical outcome for the following | Chegg.com
Predicting the Outcome of E2 Reactions Part 1 v2 – YouTube Answer and Explanation: 1 Become a Study.com member to unlock this answer! Create your account View this answer 1. The stereochemical outcome of the E2 reaction is given below. The Newman
Solved] ES Practice Problem 07.63 W Ob Identify the sole product of the… | Course Hero Stereospecificity of E2 Elimination Reactions – Chemistry Steps Ernest Zinck. It is the nature of the α carbon that determines the type of substitution. If you have a 3° carbon, the substitution reaction will be SN1. For 1° and 2° carbons, the substitution will be SN2. It is the strength of the base that determines the type of elimination. If you have a strong base, you will get E2 elimination.