Emission lines refer to the fact that glowing hot gas emits lines of light, whereas absorption lines refer to the tendency of cool atmospheric gas to absorb the same lines of light. When light passes through gas in the atmosphere some of the light at particular wavelengths is scattered resulting in darker bands. These lines came to be known as
3D Deep Learning Python Tutorial for Segmentation | Towards Data Science
When an electron jumps from higher energy state to lower energy state, it emits wavelength corresponding to energy gap of the two levels. Emission spectrum corresponds to lines or peaks on a continuous background. In the given spectrum, we can see peak in the region represented by number 1. Conclusion: Hence, the emitted lines are represented
Source Image: online.hbs.edu
Download Image
The graph above is a schematic spectrum of the planet Mars; Mars reflects visible sunlight and emits infrared light, refer to the numbered features of the graph and answer the following question.\Which of the six numbered features represents emission lines? Solution Verified Answered 1 year ago Create an account to view solutions
Source Image: mdpi.com
Download Image
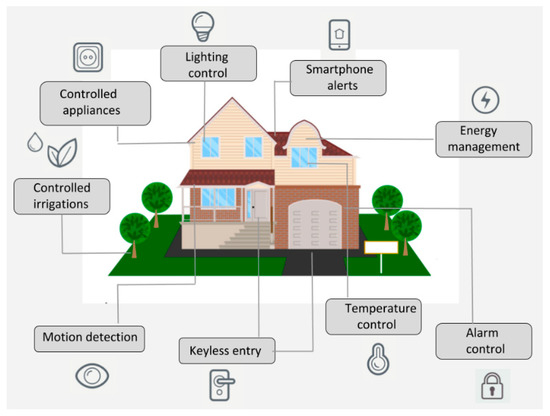
Sensors | Free Full-Text | An IoT-Based Smart Home Automation System
The conditions needed to produce line spectra. Emission and absorption lines can tell us a great deal about a distant celestial source, but they only occur under certain conditions. Emission lines from an element will appear if. there are atoms of the element present. the atoms are in a low-density gas.

Source Image: mdpi.com
Download Image
Which Of The Six Numbered Features Represents Emission Lines
The conditions needed to produce line spectra. Emission and absorption lines can tell us a great deal about a distant celestial source, but they only occur under certain conditions. Emission lines from an element will appear if. there are atoms of the element present. the atoms are in a low-density gas.
Different Graphical Representations of Spectra. Visible light spectra can be shown as images, as in the spectra below. These spectra represent energy emission as lines, with the intensity of the line (or the number of photons emitted at a particular energy) represented by the brightness and width of the line. Continuous. Emission or Bright Line.
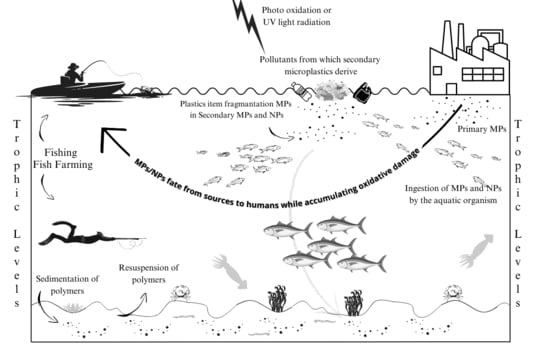
IJERPH | Free Full-Text | Assessment of Agricultural Carbon Emissions and Their Spatiotemporal Changes in China, 1997–2016
Here, in the graph pattern attached, out of the six numbered lines, the line number one represents the emission lines because the electron are releasing their energy and going back to there own ground state level. This emission when seen on the screen, it will be observed a unique pattern of the bright lines for that particular element.
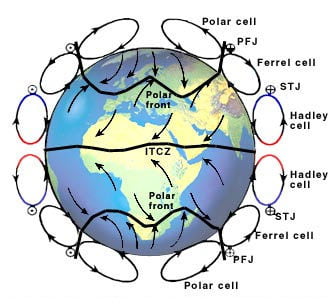
Global Atmospheric Circulation Archives – MetLink – Royal Meteorological Society
Source Image: metlink.org
Download Image
Historic variations in sea levels. Part 1: From the Holocene to Romans | Climate Etc.
Here, in the graph pattern attached, out of the six numbered lines, the line number one represents the emission lines because the electron are releasing their energy and going back to there own ground state level. This emission when seen on the screen, it will be observed a unique pattern of the bright lines for that particular element.

Source Image: judithcurry.com
Download Image
3D Deep Learning Python Tutorial for Segmentation | Towards Data Science
The graph above is a schematic spectrum of the planet Mars; Mars reflects visible sunlight and emits infrared light, refer to the numbered features of the graph and answer the following question.\Which of the six numbered features represents emission lines? Solution Verified Answered 1 year ago Create an account to view solutions

Source Image: towardsdatascience.com
Download Image
Sensors | Free Full-Text | An IoT-Based Smart Home Automation System
Emission lines refer to the fact that glowing hot gas emits lines of light, whereas absorption lines refer to the tendency of cool atmospheric gas to absorb the same lines of light. When light passes through gas in the atmosphere some of the light at particular wavelengths is scattered resulting in darker bands. These lines came to be known as
Source Image: mdpi.com
Download Image
5 Represent 6,−4,0 and 4 on a number line. 6. Fill the missing integers o..
Emission Lines and Bands. As an electron drops to a lower energy level, it loses energy by emitting a photon. The photon’s energy equals the difference in energy between its energy level and the one below it. Electrons in an atom can experience only certain, fixed changes in energy level. For instance, if an electron starts at level 3, it can
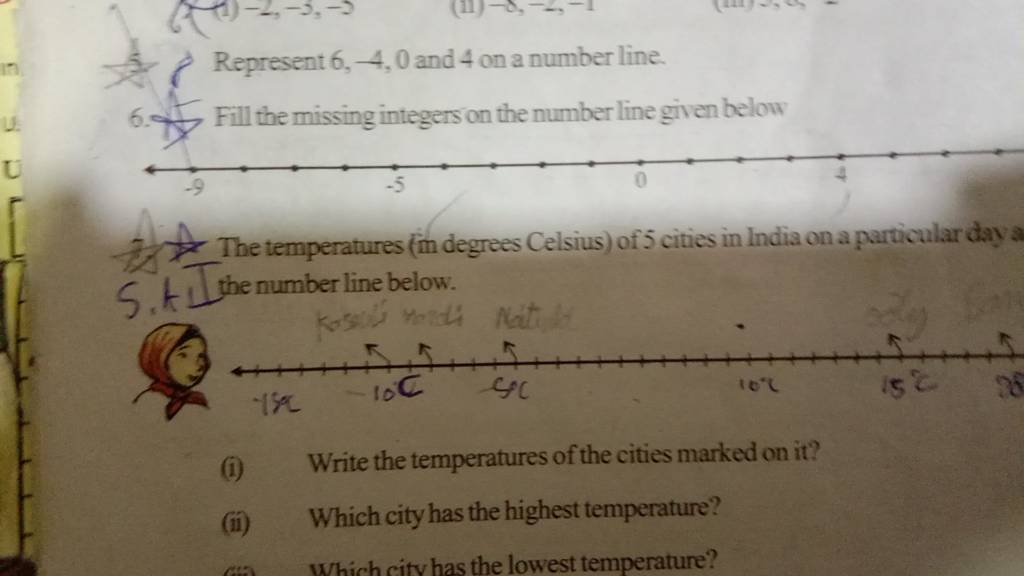
Source Image: askfilo.com
Download Image
Lesson Explainer: Emission and Absorption Spectra | Nagwa
The conditions needed to produce line spectra. Emission and absorption lines can tell us a great deal about a distant celestial source, but they only occur under certain conditions. Emission lines from an element will appear if. there are atoms of the element present. the atoms are in a low-density gas.

Source Image: nagwa.com
Download Image
Understanding DTC codes and DTC meanings | Motive
Different Graphical Representations of Spectra. Visible light spectra can be shown as images, as in the spectra below. These spectra represent energy emission as lines, with the intensity of the line (or the number of photons emitted at a particular energy) represented by the brightness and width of the line. Continuous. Emission or Bright Line.

Source Image: gomotive.com
Download Image
Historic variations in sea levels. Part 1: From the Holocene to Romans | Climate Etc.
Understanding DTC codes and DTC meanings | Motive
When an electron jumps from higher energy state to lower energy state, it emits wavelength corresponding to energy gap of the two levels. Emission spectrum corresponds to lines or peaks on a continuous background. In the given spectrum, we can see peak in the region represented by number 1. Conclusion: Hence, the emitted lines are represented
Sensors | Free Full-Text | An IoT-Based Smart Home Automation System Lesson Explainer: Emission and Absorption Spectra | Nagwa
Emission Lines and Bands. As an electron drops to a lower energy level, it loses energy by emitting a photon. The photon’s energy equals the difference in energy between its energy level and the one below it. Electrons in an atom can experience only certain, fixed changes in energy level. For instance, if an electron starts at level 3, it can